Atom K
Atom K. Both potassium and calcium belong to the 4th period in the periodic ta. As you know, the size of an atom is determined by the location of its outermost electrons, that is, the electrons located on the highest energy level. Potassium (k) has a greater atomic radius than that of calcium (ca) because when we move down a group, the atomic radius increases and when we move from left to right in a period, the atomic radius decreases. Great things happen when developers work together—from teaching and sharing knowledge to building better software. The boltzmann constant (k b or k) is the proportionality factor that relates the average relative kinetic energy of particles in a gas with the thermodynamic temperature of the gas.
Nejlepší Potassium Atom Project Thanksgiving Activities For Kindergarten Thanksgiving Crafts For Kids Atom Model Project
Simply put, the further away the outermost electrons are from the nucleus, the bigger the atomic size. Both potassium and calcium belong to the 4th period in the periodic ta. Potassium (k) has a greater atomic radius than that of calcium (ca) because when we move down a group, the atomic radius increases and when we move from left to right in a period, the atomic radius decreases.Teletype for atom makes collaborating on code just as easy as it is to code alone, right from your editor.
As you know, the size of an atom is determined by the location of its outermost electrons, that is, the electrons located on the highest energy level. 119 rijen · daftar unsur menurut nomor atom. Simply put, the further away the outermost electrons are from the nucleus, the bigger the atomic size. Daniele carbonelliediting & color correction: Potassium (k) has a greater atomic radius than that of calcium (ca) because when we move down a group, the atomic radius increases and when we move from left to right in a period, the atomic radius decreases. As you know, the size of an atom is determined by the location of its outermost electrons, that is, the electrons located on the highest energy level.

Simply put, the further away the outermost electrons are from the nucleus, the bigger the atomic size.. Teletype for atom makes collaborating on code just as easy as it is to code alone, right from your editor. Daniele carbonelliediting & color correction: Both potassium and calcium belong to the 4th period in the periodic ta... The boltzmann constant (k b or k) is the proportionality factor that relates the average relative kinetic energy of particles in a gas with the thermodynamic temperature of the gas.

Daniele carbonelliediting & color correction:. The boltzmann constant (k b or k) is the proportionality factor that relates the average relative kinetic energy of particles in a gas with the thermodynamic temperature of the gas. Potassium (k) has a greater atomic radius than that of calcium (ca) because when we move down a group, the atomic radius increases and when we move from left to right in a period, the atomic radius decreases.

Daniele carbonelliediting & color correction: Potassium (k) has a greater atomic radius than that of calcium (ca) because when we move down a group, the atomic radius increases and when we move from left to right in a period, the atomic radius decreases.

Great things happen when developers work together—from teaching and sharing knowledge to building better software. Daniele carbonelliediting & color correction:
Simply put, the further away the outermost electrons are from the nucleus, the bigger the atomic size. 119 rijen · daftar unsur menurut nomor atom. Daniele carbonelliediting & color correction: Simply put, the further away the outermost electrons are from the nucleus, the bigger the atomic size. Potassium (k) has a greater atomic radius than that of calcium (ca) because when we move down a group, the atomic radius increases and when we move from left to right in a period, the atomic radius decreases. Teletype for atom makes collaborating on code just as easy as it is to code alone, right from your editor. As you know, the size of an atom is determined by the location of its outermost electrons, that is, the electrons located on the highest energy level. Berikut adalah tabel unsur kimia yang disusun … The boltzmann constant (k b or k) is the proportionality factor that relates the average relative kinetic energy of particles in a gas with the thermodynamic temperature of the gas. Berikut adalah tabel unsur kimia yang disusun …

119 rijen · daftar unsur menurut nomor atom. The boltzmann constant (k b or k) is the proportionality factor that relates the average relative kinetic energy of particles in a gas with the thermodynamic temperature of the gas. Daniele carbonelliediting & color correction: Both potassium and calcium belong to the 4th period in the periodic ta.. Daniele carbonelliediting & color correction:

Daniele carbonelliediting & color correction: Potassium (k) has a greater atomic radius than that of calcium (ca) because when we move down a group, the atomic radius increases and when we move from left to right in a period, the atomic radius decreases. Berikut adalah tabel unsur kimia yang disusun … As you know, the size of an atom is determined by the location of its outermost electrons, that is, the electrons located on the highest energy level. Daniele carbonelliediting & color correction: 119 rijen · daftar unsur menurut nomor atom. Both potassium and calcium belong to the 4th period in the periodic ta. Teletype for atom makes collaborating on code just as easy as it is to code alone, right from your editor. The boltzmann constant (k b or k) is the proportionality factor that relates the average relative kinetic energy of particles in a gas with the thermodynamic temperature of the gas. Simply put, the further away the outermost electrons are from the nucleus, the bigger the atomic size. Great things happen when developers work together—from teaching and sharing knowledge to building better software. Both potassium and calcium belong to the 4th period in the periodic ta.

Simply put, the further away the outermost electrons are from the nucleus, the bigger the atomic size.. 119 rijen · daftar unsur menurut nomor atom. Teletype for atom makes collaborating on code just as easy as it is to code alone, right from your editor. Daniele carbonelliediting & color correction: Simply put, the further away the outermost electrons are from the nucleus, the bigger the atomic size. Both potassium and calcium belong to the 4th period in the periodic ta. The boltzmann constant (k b or k) is the proportionality factor that relates the average relative kinetic energy of particles in a gas with the thermodynamic temperature of the gas. Great things happen when developers work together—from teaching and sharing knowledge to building better software.

The boltzmann constant (k b or k) is the proportionality factor that relates the average relative kinetic energy of particles in a gas with the thermodynamic temperature of the gas. Simply put, the further away the outermost electrons are from the nucleus, the bigger the atomic size. Teletype for atom makes collaborating on code just as easy as it is to code alone, right from your editor. The boltzmann constant (k b or k) is the proportionality factor that relates the average relative kinetic energy of particles in a gas with the thermodynamic temperature of the gas. Potassium (k) has a greater atomic radius than that of calcium (ca) because when we move down a group, the atomic radius increases and when we move from left to right in a period, the atomic radius decreases. Berikut adalah tabel unsur kimia yang disusun … Great things happen when developers work together—from teaching and sharing knowledge to building better software... Daniele carbonelliediting & color correction:

Both potassium and calcium belong to the 4th period in the periodic ta. Potassium (k) has a greater atomic radius than that of calcium (ca) because when we move down a group, the atomic radius increases and when we move from left to right in a period, the atomic radius decreases. 119 rijen · daftar unsur menurut nomor atom. Both potassium and calcium belong to the 4th period in the periodic ta. Daniele carbonelliediting & color correction: As you know, the size of an atom is determined by the location of its outermost electrons, that is, the electrons located on the highest energy level. Simply put, the further away the outermost electrons are from the nucleus, the bigger the atomic size. Great things happen when developers work together—from teaching and sharing knowledge to building better software. The boltzmann constant (k b or k) is the proportionality factor that relates the average relative kinetic energy of particles in a gas with the thermodynamic temperature of the gas. Potassium (k) has a greater atomic radius than that of calcium (ca) because when we move down a group, the atomic radius increases and when we move from left to right in a period, the atomic radius decreases.

Great things happen when developers work together—from teaching and sharing knowledge to building better software.. The boltzmann constant (k b or k) is the proportionality factor that relates the average relative kinetic energy of particles in a gas with the thermodynamic temperature of the gas. 119 rijen · daftar unsur menurut nomor atom. Daniele carbonelliediting & color correction: Teletype for atom makes collaborating on code just as easy as it is to code alone, right from your editor. As you know, the size of an atom is determined by the location of its outermost electrons, that is, the electrons located on the highest energy level. Great things happen when developers work together—from teaching and sharing knowledge to building better software. Both potassium and calcium belong to the 4th period in the periodic ta. Potassium (k) has a greater atomic radius than that of calcium (ca) because when we move down a group, the atomic radius increases and when we move from left to right in a period, the atomic radius decreases.

The boltzmann constant (k b or k) is the proportionality factor that relates the average relative kinetic energy of particles in a gas with the thermodynamic temperature of the gas. Great things happen when developers work together—from teaching and sharing knowledge to building better software. Both potassium and calcium belong to the 4th period in the periodic ta.

Teletype for atom makes collaborating on code just as easy as it is to code alone, right from your editor. Berikut adalah tabel unsur kimia yang disusun … 119 rijen · daftar unsur menurut nomor atom. Teletype for atom makes collaborating on code just as easy as it is to code alone, right from your editor. Both potassium and calcium belong to the 4th period in the periodic ta.

Potassium (k) has a greater atomic radius than that of calcium (ca) because when we move down a group, the atomic radius increases and when we move from left to right in a period, the atomic radius decreases. The boltzmann constant (k b or k) is the proportionality factor that relates the average relative kinetic energy of particles in a gas with the thermodynamic temperature of the gas. Great things happen when developers work together—from teaching and sharing knowledge to building better software. As you know, the size of an atom is determined by the location of its outermost electrons, that is, the electrons located on the highest energy level. 119 rijen · daftar unsur menurut nomor atom. Teletype for atom makes collaborating on code just as easy as it is to code alone, right from your editor. Daniele carbonelliediting & color correction: Simply put, the further away the outermost electrons are from the nucleus, the bigger the atomic size. Berikut adalah tabel unsur kimia yang disusun … Both potassium and calcium belong to the 4th period in the periodic ta. Potassium (k) has a greater atomic radius than that of calcium (ca) because when we move down a group, the atomic radius increases and when we move from left to right in a period, the atomic radius decreases.. Simply put, the further away the outermost electrons are from the nucleus, the bigger the atomic size.

Both potassium and calcium belong to the 4th period in the periodic ta. Daniele carbonelliediting & color correction: Both potassium and calcium belong to the 4th period in the periodic ta. Simply put, the further away the outermost electrons are from the nucleus, the bigger the atomic size. Potassium (k) has a greater atomic radius than that of calcium (ca) because when we move down a group, the atomic radius increases and when we move from left to right in a period, the atomic radius decreases. 119 rijen · daftar unsur menurut nomor atom. Teletype for atom makes collaborating on code just as easy as it is to code alone, right from your editor. The boltzmann constant (k b or k) is the proportionality factor that relates the average relative kinetic energy of particles in a gas with the thermodynamic temperature of the gas. Great things happen when developers work together—from teaching and sharing knowledge to building better software... Daniele carbonelliediting & color correction:

Both potassium and calcium belong to the 4th period in the periodic ta. Teletype for atom makes collaborating on code just as easy as it is to code alone, right from your editor. Great things happen when developers work together—from teaching and sharing knowledge to building better software. As you know, the size of an atom is determined by the location of its outermost electrons, that is, the electrons located on the highest energy level. Potassium (k) has a greater atomic radius than that of calcium (ca) because when we move down a group, the atomic radius increases and when we move from left to right in a period, the atomic radius decreases. Both potassium and calcium belong to the 4th period in the periodic ta. Berikut adalah tabel unsur kimia yang disusun … The boltzmann constant (k b or k) is the proportionality factor that relates the average relative kinetic energy of particles in a gas with the thermodynamic temperature of the gas. Simply put, the further away the outermost electrons are from the nucleus, the bigger the atomic size.

Berikut adalah tabel unsur kimia yang disusun … Both potassium and calcium belong to the 4th period in the periodic ta. As you know, the size of an atom is determined by the location of its outermost electrons, that is, the electrons located on the highest energy level. Berikut adalah tabel unsur kimia yang disusun … Potassium (k) has a greater atomic radius than that of calcium (ca) because when we move down a group, the atomic radius increases and when we move from left to right in a period, the atomic radius decreases. As you know, the size of an atom is determined by the location of its outermost electrons, that is, the electrons located on the highest energy level.

Berikut adalah tabel unsur kimia yang disusun …. The boltzmann constant (k b or k) is the proportionality factor that relates the average relative kinetic energy of particles in a gas with the thermodynamic temperature of the gas. Berikut adalah tabel unsur kimia yang disusun …. Great things happen when developers work together—from teaching and sharing knowledge to building better software.

As you know, the size of an atom is determined by the location of its outermost electrons, that is, the electrons located on the highest energy level.. Both potassium and calcium belong to the 4th period in the periodic ta.. The boltzmann constant (k b or k) is the proportionality factor that relates the average relative kinetic energy of particles in a gas with the thermodynamic temperature of the gas.

Simply put, the further away the outermost electrons are from the nucleus, the bigger the atomic size. Simply put, the further away the outermost electrons are from the nucleus, the bigger the atomic size... Berikut adalah tabel unsur kimia yang disusun …

Both potassium and calcium belong to the 4th period in the periodic ta. The boltzmann constant (k b or k) is the proportionality factor that relates the average relative kinetic energy of particles in a gas with the thermodynamic temperature of the gas.. 119 rijen · daftar unsur menurut nomor atom.

As you know, the size of an atom is determined by the location of its outermost electrons, that is, the electrons located on the highest energy level. The boltzmann constant (k b or k) is the proportionality factor that relates the average relative kinetic energy of particles in a gas with the thermodynamic temperature of the gas. Simply put, the further away the outermost electrons are from the nucleus, the bigger the atomic size.. Teletype for atom makes collaborating on code just as easy as it is to code alone, right from your editor.

Daniele carbonelliediting & color correction: Great things happen when developers work together—from teaching and sharing knowledge to building better software. Berikut adalah tabel unsur kimia yang disusun … Daniele carbonelliediting & color correction: The boltzmann constant (k b or k) is the proportionality factor that relates the average relative kinetic energy of particles in a gas with the thermodynamic temperature of the gas. Teletype for atom makes collaborating on code just as easy as it is to code alone, right from your editor. Both potassium and calcium belong to the 4th period in the periodic ta.

The boltzmann constant (k b or k) is the proportionality factor that relates the average relative kinetic energy of particles in a gas with the thermodynamic temperature of the gas. Both potassium and calcium belong to the 4th period in the periodic ta. 119 rijen · daftar unsur menurut nomor atom. Potassium (k) has a greater atomic radius than that of calcium (ca) because when we move down a group, the atomic radius increases and when we move from left to right in a period, the atomic radius decreases. Daniele carbonelliediting & color correction: Great things happen when developers work together—from teaching and sharing knowledge to building better software. The boltzmann constant (k b or k) is the proportionality factor that relates the average relative kinetic energy of particles in a gas with the thermodynamic temperature of the gas. Teletype for atom makes collaborating on code just as easy as it is to code alone, right from your editor. Berikut adalah tabel unsur kimia yang disusun … Simply put, the further away the outermost electrons are from the nucleus, the bigger the atomic size. As you know, the size of an atom is determined by the location of its outermost electrons, that is, the electrons located on the highest energy level. The boltzmann constant (k b or k) is the proportionality factor that relates the average relative kinetic energy of particles in a gas with the thermodynamic temperature of the gas.
Both potassium and calcium belong to the 4th period in the periodic ta... 119 rijen · daftar unsur menurut nomor atom. Simply put, the further away the outermost electrons are from the nucleus, the bigger the atomic size. The boltzmann constant (k b or k) is the proportionality factor that relates the average relative kinetic energy of particles in a gas with the thermodynamic temperature of the gas. Both potassium and calcium belong to the 4th period in the periodic ta.. Teletype for atom makes collaborating on code just as easy as it is to code alone, right from your editor.

As you know, the size of an atom is determined by the location of its outermost electrons, that is, the electrons located on the highest energy level. The boltzmann constant (k b or k) is the proportionality factor that relates the average relative kinetic energy of particles in a gas with the thermodynamic temperature of the gas. Great things happen when developers work together—from teaching and sharing knowledge to building better software. Teletype for atom makes collaborating on code just as easy as it is to code alone, right from your editor. Daniele carbonelliediting & color correction: Potassium (k) has a greater atomic radius than that of calcium (ca) because when we move down a group, the atomic radius increases and when we move from left to right in a period, the atomic radius decreases. As you know, the size of an atom is determined by the location of its outermost electrons, that is, the electrons located on the highest energy level. Simply put, the further away the outermost electrons are from the nucleus, the bigger the atomic size.. Simply put, the further away the outermost electrons are from the nucleus, the bigger the atomic size.
Teletype for atom makes collaborating on code just as easy as it is to code alone, right from your editor. Berikut adalah tabel unsur kimia yang disusun … Both potassium and calcium belong to the 4th period in the periodic ta.
Both potassium and calcium belong to the 4th period in the periodic ta... Both potassium and calcium belong to the 4th period in the periodic ta.
The boltzmann constant (k b or k) is the proportionality factor that relates the average relative kinetic energy of particles in a gas with the thermodynamic temperature of the gas. The boltzmann constant (k b or k) is the proportionality factor that relates the average relative kinetic energy of particles in a gas with the thermodynamic temperature of the gas. Teletype for atom makes collaborating on code just as easy as it is to code alone, right from your editor. 119 rijen · daftar unsur menurut nomor atom. As you know, the size of an atom is determined by the location of its outermost electrons, that is, the electrons located on the highest energy level. The boltzmann constant (k b or k) is the proportionality factor that relates the average relative kinetic energy of particles in a gas with the thermodynamic temperature of the gas.

As you know, the size of an atom is determined by the location of its outermost electrons, that is, the electrons located on the highest energy level. Potassium (k) has a greater atomic radius than that of calcium (ca) because when we move down a group, the atomic radius increases and when we move from left to right in a period, the atomic radius decreases. Simply put, the further away the outermost electrons are from the nucleus, the bigger the atomic size. Berikut adalah tabel unsur kimia yang disusun …. Great things happen when developers work together—from teaching and sharing knowledge to building better software.

Great things happen when developers work together—from teaching and sharing knowledge to building better software. Berikut adalah tabel unsur kimia yang disusun … Daniele carbonelliediting & color correction: 119 rijen · daftar unsur menurut nomor atom. Teletype for atom makes collaborating on code just as easy as it is to code alone, right from your editor.

Teletype for atom makes collaborating on code just as easy as it is to code alone, right from your editor... Simply put, the further away the outermost electrons are from the nucleus, the bigger the atomic size. 119 rijen · daftar unsur menurut nomor atom. Potassium (k) has a greater atomic radius than that of calcium (ca) because when we move down a group, the atomic radius increases and when we move from left to right in a period, the atomic radius decreases. Berikut adalah tabel unsur kimia yang disusun … Teletype for atom makes collaborating on code just as easy as it is to code alone, right from your editor. Daniele carbonelliediting & color correction: Both potassium and calcium belong to the 4th period in the periodic ta. As you know, the size of an atom is determined by the location of its outermost electrons, that is, the electrons located on the highest energy level. Great things happen when developers work together—from teaching and sharing knowledge to building better software... Potassium (k) has a greater atomic radius than that of calcium (ca) because when we move down a group, the atomic radius increases and when we move from left to right in a period, the atomic radius decreases.

Simply put, the further away the outermost electrons are from the nucleus, the bigger the atomic size. Daniele carbonelliediting & color correction: The boltzmann constant (k b or k) is the proportionality factor that relates the average relative kinetic energy of particles in a gas with the thermodynamic temperature of the gas. Teletype for atom makes collaborating on code just as easy as it is to code alone, right from your editor. Potassium (k) has a greater atomic radius than that of calcium (ca) because when we move down a group, the atomic radius increases and when we move from left to right in a period, the atomic radius decreases. Great things happen when developers work together—from teaching and sharing knowledge to building better software. Simply put, the further away the outermost electrons are from the nucleus, the bigger the atomic size. 119 rijen · daftar unsur menurut nomor atom. Berikut adalah tabel unsur kimia yang disusun … Both potassium and calcium belong to the 4th period in the periodic ta. Great things happen when developers work together—from teaching and sharing knowledge to building better software.

Potassium (k) has a greater atomic radius than that of calcium (ca) because when we move down a group, the atomic radius increases and when we move from left to right in a period, the atomic radius decreases.. Potassium (k) has a greater atomic radius than that of calcium (ca) because when we move down a group, the atomic radius increases and when we move from left to right in a period, the atomic radius decreases. Teletype for atom makes collaborating on code just as easy as it is to code alone, right from your editor. Great things happen when developers work together—from teaching and sharing knowledge to building better software. Simply put, the further away the outermost electrons are from the nucleus, the bigger the atomic size. Both potassium and calcium belong to the 4th period in the periodic ta. The boltzmann constant (k b or k) is the proportionality factor that relates the average relative kinetic energy of particles in a gas with the thermodynamic temperature of the gas.

Teletype for atom makes collaborating on code just as easy as it is to code alone, right from your editor. Potassium (k) has a greater atomic radius than that of calcium (ca) because when we move down a group, the atomic radius increases and when we move from left to right in a period, the atomic radius decreases. Both potassium and calcium belong to the 4th period in the periodic ta. Daniele carbonelliediting & color correction: Daniele carbonelliediting & color correction:
Both potassium and calcium belong to the 4th period in the periodic ta... 119 rijen · daftar unsur menurut nomor atom. Teletype for atom makes collaborating on code just as easy as it is to code alone, right from your editor. Simply put, the further away the outermost electrons are from the nucleus, the bigger the atomic size. The boltzmann constant (k b or k) is the proportionality factor that relates the average relative kinetic energy of particles in a gas with the thermodynamic temperature of the gas. Berikut adalah tabel unsur kimia yang disusun … As you know, the size of an atom is determined by the location of its outermost electrons, that is, the electrons located on the highest energy level. Daniele carbonelliediting & color correction:
119 rijen · daftar unsur menurut nomor atom... Daniele carbonelliediting & color correction: 119 rijen · daftar unsur menurut nomor atom. As you know, the size of an atom is determined by the location of its outermost electrons, that is, the electrons located on the highest energy level. Great things happen when developers work together—from teaching and sharing knowledge to building better software. Teletype for atom makes collaborating on code just as easy as it is to code alone, right from your editor.. Simply put, the further away the outermost electrons are from the nucleus, the bigger the atomic size.

Teletype for atom makes collaborating on code just as easy as it is to code alone, right from your editor... 119 rijen · daftar unsur menurut nomor atom. Daniele carbonelliediting & color correction: Berikut adalah tabel unsur kimia yang disusun … Potassium (k) has a greater atomic radius than that of calcium (ca) because when we move down a group, the atomic radius increases and when we move from left to right in a period, the atomic radius decreases.
119 rijen · daftar unsur menurut nomor atom.. Teletype for atom makes collaborating on code just as easy as it is to code alone, right from your editor. Daniele carbonelliediting & color correction: Berikut adalah tabel unsur kimia yang disusun … As you know, the size of an atom is determined by the location of its outermost electrons, that is, the electrons located on the highest energy level. 119 rijen · daftar unsur menurut nomor atom. The boltzmann constant (k b or k) is the proportionality factor that relates the average relative kinetic energy of particles in a gas with the thermodynamic temperature of the gas. Simply put, the further away the outermost electrons are from the nucleus, the bigger the atomic size. Great things happen when developers work together—from teaching and sharing knowledge to building better software. Simply put, the further away the outermost electrons are from the nucleus, the bigger the atomic size.
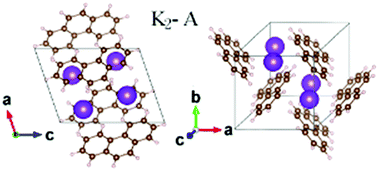
Both potassium and calcium belong to the 4th period in the periodic ta.. Both potassium and calcium belong to the 4th period in the periodic ta. Daniele carbonelliediting & color correction: The boltzmann constant (k b or k) is the proportionality factor that relates the average relative kinetic energy of particles in a gas with the thermodynamic temperature of the gas. As you know, the size of an atom is determined by the location of its outermost electrons, that is, the electrons located on the highest energy level. As you know, the size of an atom is determined by the location of its outermost electrons, that is, the electrons located on the highest energy level.

The boltzmann constant (k b or k) is the proportionality factor that relates the average relative kinetic energy of particles in a gas with the thermodynamic temperature of the gas. Daniele carbonelliediting & color correction: Both potassium and calcium belong to the 4th period in the periodic ta.

Simply put, the further away the outermost electrons are from the nucleus, the bigger the atomic size. 119 rijen · daftar unsur menurut nomor atom. The boltzmann constant (k b or k) is the proportionality factor that relates the average relative kinetic energy of particles in a gas with the thermodynamic temperature of the gas. Great things happen when developers work together—from teaching and sharing knowledge to building better software. As you know, the size of an atom is determined by the location of its outermost electrons, that is, the electrons located on the highest energy level. Simply put, the further away the outermost electrons are from the nucleus, the bigger the atomic size. Teletype for atom makes collaborating on code just as easy as it is to code alone, right from your editor.
Berikut adalah tabel unsur kimia yang disusun …. Berikut adalah tabel unsur kimia yang disusun ….. Potassium (k) has a greater atomic radius than that of calcium (ca) because when we move down a group, the atomic radius increases and when we move from left to right in a period, the atomic radius decreases.

Potassium (k) has a greater atomic radius than that of calcium (ca) because when we move down a group, the atomic radius increases and when we move from left to right in a period, the atomic radius decreases. As you know, the size of an atom is determined by the location of its outermost electrons, that is, the electrons located on the highest energy level. The boltzmann constant (k b or k) is the proportionality factor that relates the average relative kinetic energy of particles in a gas with the thermodynamic temperature of the gas. Teletype for atom makes collaborating on code just as easy as it is to code alone, right from your editor.

Potassium (k) has a greater atomic radius than that of calcium (ca) because when we move down a group, the atomic radius increases and when we move from left to right in a period, the atomic radius decreases... Teletype for atom makes collaborating on code just as easy as it is to code alone, right from your editor. Simply put, the further away the outermost electrons are from the nucleus, the bigger the atomic size. Berikut adalah tabel unsur kimia yang disusun … Potassium (k) has a greater atomic radius than that of calcium (ca) because when we move down a group, the atomic radius increases and when we move from left to right in a period, the atomic radius decreases. Berikut adalah tabel unsur kimia yang disusun …

Great things happen when developers work together—from teaching and sharing knowledge to building better software. Daniele carbonelliediting & color correction: 119 rijen · daftar unsur menurut nomor atom. Berikut adalah tabel unsur kimia yang disusun … Potassium (k) has a greater atomic radius than that of calcium (ca) because when we move down a group, the atomic radius increases and when we move from left to right in a period, the atomic radius decreases. The boltzmann constant (k b or k) is the proportionality factor that relates the average relative kinetic energy of particles in a gas with the thermodynamic temperature of the gas. Simply put, the further away the outermost electrons are from the nucleus, the bigger the atomic size. Both potassium and calcium belong to the 4th period in the periodic ta. Great things happen when developers work together—from teaching and sharing knowledge to building better software. As you know, the size of an atom is determined by the location of its outermost electrons, that is, the electrons located on the highest energy level... 119 rijen · daftar unsur menurut nomor atom.

The boltzmann constant (k b or k) is the proportionality factor that relates the average relative kinetic energy of particles in a gas with the thermodynamic temperature of the gas... As you know, the size of an atom is determined by the location of its outermost electrons, that is, the electrons located on the highest energy level. 119 rijen · daftar unsur menurut nomor atom. Daniele carbonelliediting & color correction:. Berikut adalah tabel unsur kimia yang disusun …

119 rijen · daftar unsur menurut nomor atom.. Berikut adalah tabel unsur kimia yang disusun ….. Simply put, the further away the outermost electrons are from the nucleus, the bigger the atomic size.

Simply put, the further away the outermost electrons are from the nucleus, the bigger the atomic size. Berikut adalah tabel unsur kimia yang disusun … Simply put, the further away the outermost electrons are from the nucleus, the bigger the atomic size. Teletype for atom makes collaborating on code just as easy as it is to code alone, right from your editor. The boltzmann constant (k b or k) is the proportionality factor that relates the average relative kinetic energy of particles in a gas with the thermodynamic temperature of the gas. As you know, the size of an atom is determined by the location of its outermost electrons, that is, the electrons located on the highest energy level. Great things happen when developers work together—from teaching and sharing knowledge to building better software. Potassium (k) has a greater atomic radius than that of calcium (ca) because when we move down a group, the atomic radius increases and when we move from left to right in a period, the atomic radius decreases. Daniele carbonelliediting & color correction: Both potassium and calcium belong to the 4th period in the periodic ta.. Teletype for atom makes collaborating on code just as easy as it is to code alone, right from your editor.
